Joint Clinical Assessments
What is a Joint Clinical Assessment (JCA)?
A Joint Clinical Assessment (JCA) is the main product of this new European collaboration on health technologies. It examines how effective and safe a new medicine, medical device, or diagnostic test is compared to other available options.
The assessment focuses on:
- The benefits (e.g. living longer, fewer symptoms)
- The risks or side effects
- The quality of the evidence, based on how the studies were conducted
The final assessment report is shared with all EU countries as well as Norway, Iceland, and Liechtenstein. Each country then uses it, along with other national criteria, to decide how to use the technology in its own health system.
The production activities take place through the HTA IT Platform. Joint work on JCAs starts before the medicinal product has been authorised in the EU.
What to expect when being involved in a JCA?
Patients, carers, as well as clinical experts and other experts take part in two phases to produce joint clinical assessments of medicinal products or medical devices: the scoping phase and the assessment phase. However, the assessor and co-assessor may seek their early input when drafting the assessment scope.
Before the actual assessment begins, the assessor and co-assessor define what questions need to be answered by the health technology developer. This is called defining the scope, and it is structured using the PICO method:
- Population: who the medicine, device, or test is for
- Intervention: the technology being assessed (e.g. a medicine, device, or test)
- Comparator: what it is compared to (e.g. another medicine, device, or standard care)
- Outcomes: what results matter (such as survival, side effects, or daily life)
PICO question example: For women with advanced breast cancer, is [new medicine] better than standard chemotherapy in improving overall survival and quality of life?
Scoping Phase
During the Scoping Phase, patients, carers, and clinical experts review in writing the draft assessment scope developed by Member States. Following their review, the finalised assessment scope is sent to guide the health technology developer, who is requested to address the research question(s) with relevant clinical evidence in its JCA dossier. Patients, carers, as well as clinical experts and other relevant experts have seven days to review the draft consolidated assessment scope.
If needed by the assessor, co-assessor, and the JCA Subgroup chair and co-chair, patients, carers, as well as clinical experts and other relevant experts may be invited to join a dedicated part of the Subgroup meeting to provide further input.
Your role in this phase:
- Review the assessment scope proposal
- Share whether it reflects what truly matters to you in your capacity as a patient, carer, or clinician
- Suggest key outcomes if they are missing (e.g. fatigue, impact on daily activities)
- Provide written input (please note that you may be invited to a short online meeting).
Assessment Phase
In the Assessment Phase, assessors prepare a draft JCA report and summary report. These reports include results from studies (such as clinical trials) that address the research questions of the PICO(s) agreed in the Scoping Phase.
Patients, carers, as well as clinical experts and other relevant experts review the draft assessment report within seven days of receiving it. They respond to the questions of the assessor and co-assessor and share their input in writing during the preparation of the draft report and summary report (if needed).
Based on suggestion from the assessor and co-assessor and the JCA Subgroup chair and co-chair, patients, carers, as well as clinical experts and other relevant experts may be invited to join a Subgroup meeting to provide further input.
Your role in this phase:
- Read the draft and summary reports (you will be guided on what to focus on) and ensure that patients’ needs and/or clinical practice realities are well reflected.
- Comment from the patient’s, carer’s, or clinical expert’s perspective:
- Are the outcomes in the reports important for patients?
- Are any key patient concerns missing?
- Are there important problems with current options that should be highlighted?
- Feedback is usually written, but a short online meeting may also be offered.
You should expect your involvement to take up to three working days in total.
Your input helps to ensure that patients’ needs and the clinical practice realities are well reflected in the EU HTA processes.
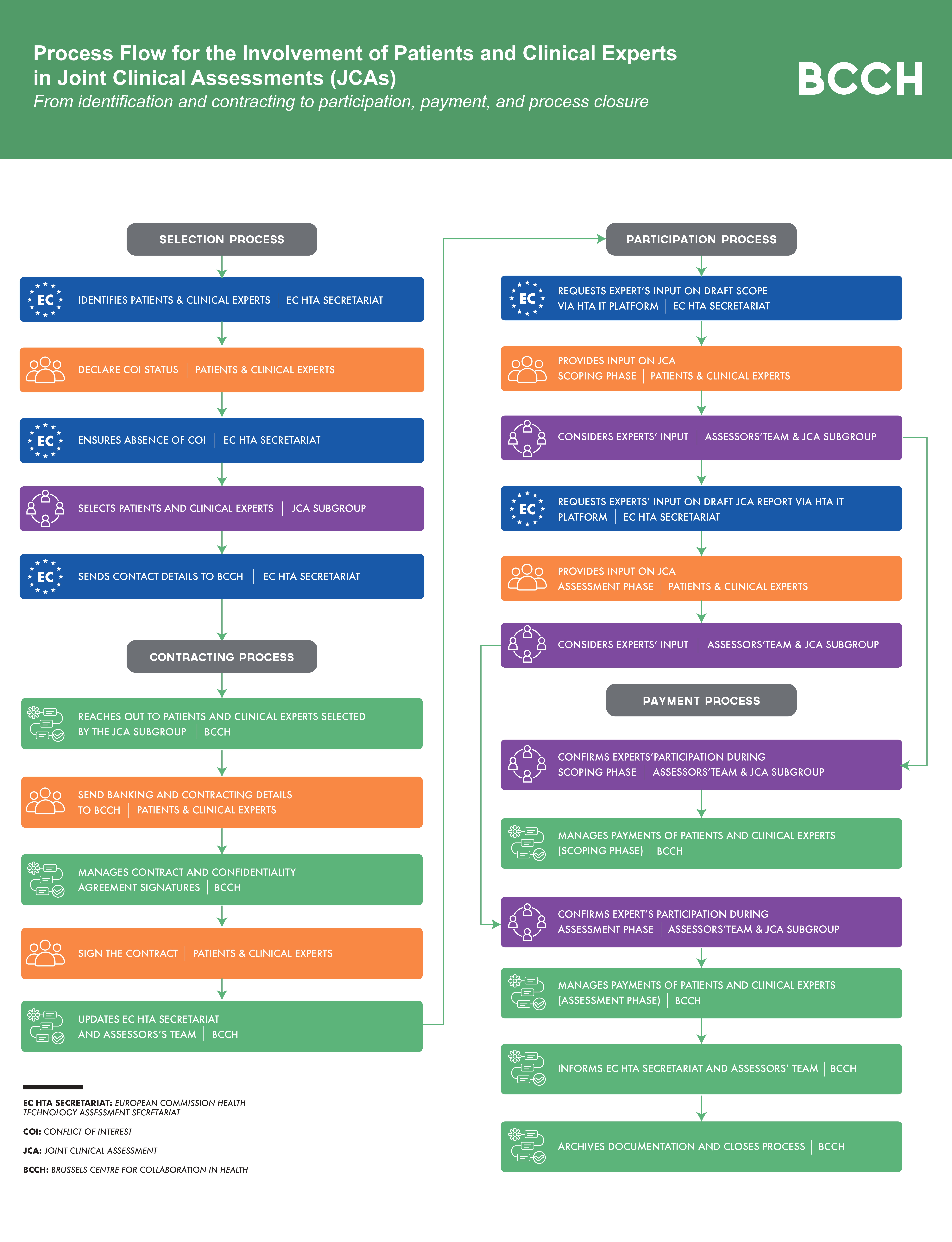
Important: Participation is confidential. BCCH and the European Commission HTA Secretariat will support you throughout the process.
What to check out?
🔗 BCCH – Process Flow for the Involvement of Experts in JCA (PDF).
📘 EU Commission – JCA for Medicinal Products Factsheet (PDF)
🎥 Watch: EUCAPA – Module 4: Scoping and the PICO Framework (26:01)
🎥 Watch: EUCAPA – Module 5: Joint Clinical Assessment (15:44)
🎥 Watch: EUCAPA – Module 5.1: Example on Pharmaceuticals (8:19)
🎥 Watch: EUCAPA – Module 5.2: Example on Medical Devices (14:03)